What is TIDE BC?
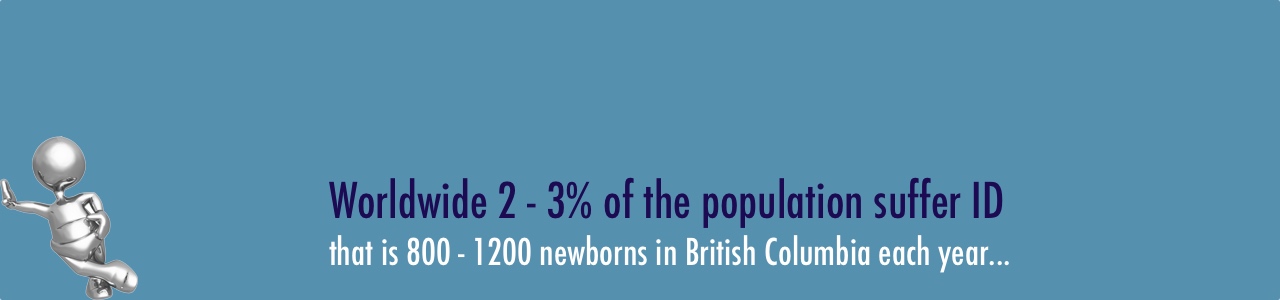
Tide BC is a new care & research initiative with a focus on prevention and treatment of Intellectual Disability (ID). We have shown that the ID seen in some children is due to treatable genetic conditions known as inborn errors of metabolism. Many of these can be treated with diet or drugs.
Our Goal?
By improving diagnosis, treatment and care and by using the best of modern day technologies, TIDE BC aims to improve health outcomes of all those affected.
TIDE BC has developed an evidence based protocol designed to effectively identify treatable inborn errors of metabolism in patients with global developmental delay / intellectual disability.
The TIDE team has developed and implemented novel treatments for inborn errors of metabolism presenting with intellectual developmental disability.
Our TIDEX project aimed to identify novel (potentially treatable) ID genes employing the utility of the metabolic phenotype. Our success rate emphasizes the advantages of the biochemical phenotype for gene discovery.
Each patient has a unique story. The stories of some of the patients that have been participating in the TIDE study are documented in this section.